Cooling Towers
Efficient cooling tower treatment with ozone
Cooling towers are a relatively new sector for ozone treatment. As such, the benefits of the technology are still being discovered by the users in this sector. The main advantages of ozone water treatment over the traditional chemical water treatment are in the water and energy savings that can be made. The reduction, and possible elimination, of chemical use also creates cost benefits for the user.
The first problem facing water cooling towers is the build up of biological growth and minerals, otherwise known as scale. These problems inhibit the cooling towers’ heat transfer efficiency. The way this problem has been solved in the past has been through the use of chemical agents such as chlorine and chelating agents. While this serves as an adequate solution to the original problem, the chemicals lead to other problems. Because of the evaporation of water in the tower, the remaining water reaches a high level of chemical and contaminant concentration. To regulate this, water is bled out of the system, and replaced by fresh “make up” water. It is the bleed off water that can be problematic to dispose of, with extra sewage cost being incurred.
Ozone treatment solves the original problem with a vastly reduced number of secondary costs and considerations. As well as being a powerful biocide, killing virus and infectious bacteria, ozone has been proven to have a positive de-scaling effect. It also greatly reduces the level of bleed off water, as well as the per unit cost of disposing it due to the environmentally friendly nature of ozone. Added to this are the savings due to reduced storage costs and handling of chemicals as ozone is produced on site. This fact significantly simplifies regulatory compliance.
Why use ozone treatment?
By implementing ozonation water treatment for cooling towers three main types of savings can be made due to:
- Increased cooling operation efficiency (which lowers power consumption).
- Reduced blowdown amount (reducing costs from makeup water and chemical waste discharge).
- Reduced maintenance costs. Maintenance labor costs for ozonation treatment systems are minor.
- Insignificant buildup of disinfectant or disinfectant byproducts
- Very effective disinfectant
- No need for handling of hazardous chemicals due to in-situ production
- Low corrosion
- Environmentally friendly treatment, facilitating regulatory compliance
For sites operating their own water and sewage treatment facilities some concrete benefits are listed below:
- Reduced pumping power to extract and transport water from reservoir to water treatment facility due to decreased makeup water consumption
- Reduced chemical, filtration, and maintenance costs
- Reduced pumping power for blowdown transportation to sewage treatment
- Reduced pumping power for water transportation from water treatment to end-user
- Reduced permit costs for discharge of treated water to environment
Ozone treatment potential
Quoting the U.S. Department of Energy Federal Technology Alert on ozone treatment:
In a properly installed and operating system, bacterial counts are reduced, with subsequent minimization of biofilm buildup on heat exchanger surfaces. The reduction in energy demand, the increased operating efficiency, and the reduced maintenance effort provide cost savings as well as environmental benefits and improved regulatory compliance with respect to discharge of wastewater from blowdown.
The ozone mechanism
Ozone effectively inactivates and kills microorganisms by oxidizing their organic constituents and rupturing the cell walls. It is a biocidal process to which microbes cannot develop immunity. For example a 0.4 mg/L concentration results in 100 % kill in 2 – 3 minutes for the biofilm producer Pseudomonas fluorescens. A 0.1 mg/L concentration will remove about 80 % of the biofilm in 3 hours.
Ozonation technology also has beneficial scaling treatment effects. By removing biofilm to which scale is adhered scaling effects can be significantly reduced when biofilm is present.
Low corrosive effects
Corrosion effects are a usual common concern when using ozone. However, because a very low concentration is required and the short half-life, corrosive effects of ozone are low (or even half that resulting from chlorination treatment). Moreover, the effectiveness as a biocide minimizes significant corrosion effects induced by microbiological activity. Also, ozone treatment has been shown to increase the corrosion protection by forming a passive film covering and protecting the exposed surface.
Case study data
Case studies show that typical turnkey costs for ozonation systems required to treat a 1000 ton (3.5 MW) range from $40,000 to $50,000. In a case study (made by the U.S. Department of Energy) in -94 at a Lockheed Martin Facility in Florida the ozonation system could be installed in one day, eventually resulting in a 90 % blowdown waste reduction and a savings to investment ratio (SIR) of 31.2. Furthermore it was shown that the feared corrosion effect from using ozone was only half that resulting from chlorine treatment. The annual operation cost comparison for the Lockheed Martin factory is shown in the table below.
| Item | Chemical Treatment | Ozone Treatment |
|---|---|---|
| Electrical operation | $0 | $2,592 |
| Chemicals | $18,613 | $0 |
| Labor | $9,360 | $2,808 |
| Blowdown Hauling | $45,360 | $4,536 |
| Chlorine gas | $6,120 | $0 |
| Power consumption | $118,715 | $47,479 |
| Total cost/year | $198,168 | $57,415 |
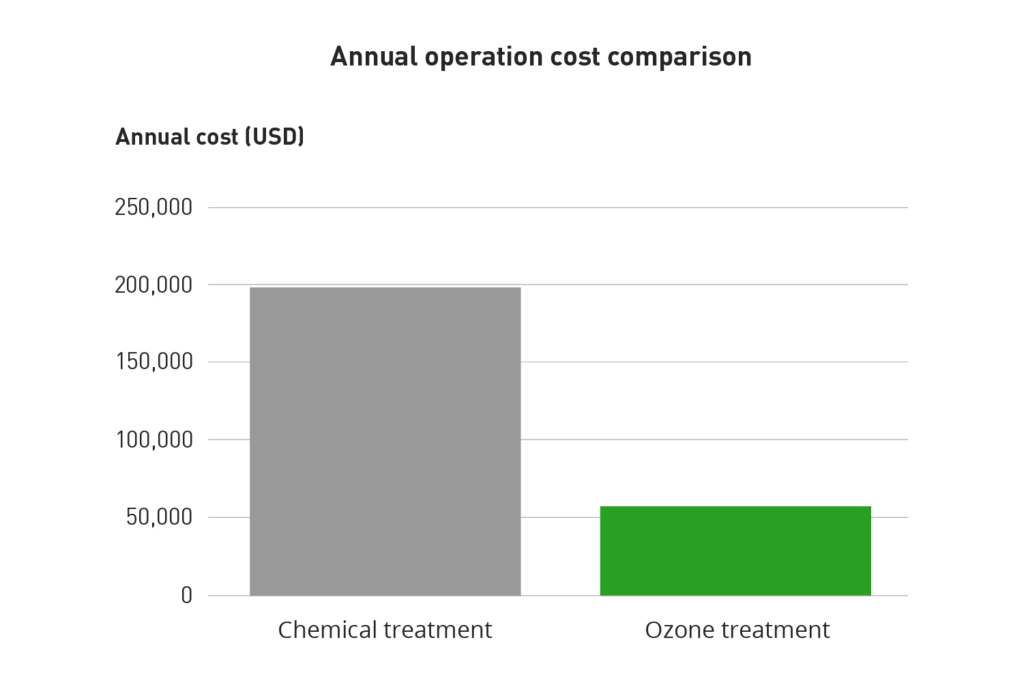
Cost comparison for chemical and ozone treatment.
Important parameters to consider when using ozone treatment
- Preparation of inlet air to ozone generator. To maximize life time and capacity of the ozone generator a dry, concentrated air feed should be supplied.
- Adequate dosing and capacity of ozone generator
- Efficient ozone generator cooling. This is also critical to achieve long life times and capacity of the generator.
- Harder to use where high COD-levels are introduced to the water from makeup or local air conditions. This consumes the main part of the ozone. For example, this is the reason why ozone treatment is more difficult in some chemical- and petrochemical plants where organic material is introduced to the system from the air.
- A makeup water quality of above 150 ppm calcium hardness may require a side stream filter. Calcium (CaCO3) hardness of above 500 ppm or sulfates above 100 ppm should not be considered for ozone treatment.
- Water temperature. The cooling water temperature should not exceed 45 ⁰C for efficient ozone treatment. This is mainly because of the low solubility of ozone at higher temperatures.
- Long piping systems. Because of the short half life time of about 10 – 15 multiple injection points may be required in cooling towers larger than about 400 m3.
- Use ozone compatible materials and monitor corrosion (e.g. using corrosion coupons).
Main contaminant effects
As already stated four main issues appear when circulating cooling tower water, namely corrosion, scale formation, biofouling, and pathogenic growth.

Visual display of contaminant effects
The main four main contaminant effects as well as their respective treatments are briefly described in the table below:
| Corrosion | Corrosion generally appear in water contacting applications due to oxidation reactions. This leads to structural and equipment damage which affects performance and lifetime of the process. Addition of corrosive chemicals enhance these effects. |
| – Treatment | While possible to control, corrosion is basically impossible to avoid completely. Again, different makeup water qualities require different treatments. However it is important to note that the corrosion effects are severe when using soft or softened makeup water. |
| Scaling | The formation of scale leads to two major issues, namely fluid flow obstruction and significantly decreased heat transfer efficiency. The conductivity of for example copper is more than 400 times that of calcium carbonate. For instance, a 1.5 mil or 0.025 mm layer of calcium carbonate decreases the heat transfer efficiency by about 12.5 %. |
| – Treatment | Scaling is treated using different approaches. Scaling inhibition chemicals can be used either to adsorb minerals on growing crystals or to convert scale forming ions into non-scale forming compounds. Another approach involves lowering the pH by acid addition which dissolves the scale. Finally scaling effects may also be mitigated by adding softened makeup water. |
| Biofouling | Biofouling shows similar negative effects as scaling but with an even lower conductivity than calcium carbonate scale. Hence, it is important to manage water quality both with respect to mineral content and microorganisms. |
| – Treatment | Oxidizing and non-oxidizing biocides (see description below). |
| Pathogens | Pathogenic outbreaks in coolant water circuits is a common issue which leads to infection risk in the vicinity of the cooling facility. The pathogens can be transported to the surroundings together with the evaporating stream. In 2004 an outbreak of Legionella was reported in Pas-de-Calais in France were bacteria were found up to 6 km from a cooling tower, which was the source of the outbreak. The outbreak killed 21 of 86 people with laboratory confirmed infection. |
| – Treatment | Oxidizing and non-oxidizing biocides (see description below). |
Biological treatment – biocides
The biocidal function is very important in the cooling tower system as it is continuously exposed to airborne organic material and organisms. Biocides to control microbiological growth (to prevent both biofouling and pathogens) may be divided into two types, namely oxidizing and non-oxidizing biocides.
Oxidizing biocides
In general oxidizing biocides prove to be effective disinfectants which oxidize and therefore kill the microorganisms rapidly at low dosage. General drawbacks for some of these compounds include: reduction of pH level, increased corrosion, and sensitivity to pH alterations. Ozone is an oxidizing biocide with insignificant negative effects when handled professionally.
Non-oxidizing biocides
Non-oxidizing biocides function by stressing the microbes and interfering with their metabolic mechanisms which eventually deactivates them. Because of this, some microorganisms may develop resistance towards non-oxidizing agents which leads to replacement of one type of microbe by another. Therefore non-oxidizing agents should be used in combination with other non-oxidizing or oxidizing agents. Moreover non-oxidizing biocides generally require high dosage, long contact time, and are relatively expensive. What does work to their advantage is their ability to target specific types of microbes as well as their non-corrosive properties.
Biocide examples
Presented in the table below are examples of oxidizing and non-oxidizing biocides that have been or are currently employed to control microbial growth.
| Oxidizing biocides | Non-oxidizing biocides |
|---|---|
Benzalkonium chloride, a so-called “quat”
Water hardness and scaling
The scaling problem essentially arises from mineral concentration buildup or in other words increasing hardness of the water. Multivalent cations, mainly Ca2+ and Mg2+, and carbonates are the main sources of water hardness. One of the most important pathways for scaling buildup is expressed below in the following chemical equilibrium reaction:
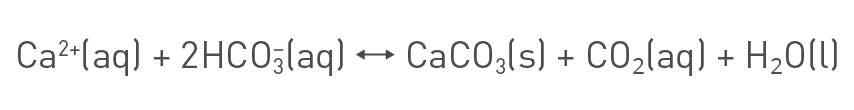
Calcium carbonate equilibrium reaction.
As a result of equilibrium reactions like the one above, a higher dissolved mineral content will lead to an increased formation of solid mineral salts, or expressed differently, scaling.
A second pathway for scaling buildup is via biological mineral deposition on biofilms. Biofilms have been shown to function as an adherent for mineral microcrystals. This way, formation of biofilm also promotes scaling buildup.

Depósito de una incrustación de cal (carbonato cálcico)
Cycles of concentration
A general issue of cooling tower operation is the buildup of dissolved mineral concentration. In short this build-up is caused when coolant water is evaporated and the mineral content is retained in solution. When the mineral content reaches above the solubility level precipitation of the minerals occur. This in turn leads to a gradual build-up of scale deposits. To control the mineral content a portion of the coolant stream is bled off and replaced by a fresh makeup water source. Additionally scaling inhibitor chemicals are used to increase the solubility of the minerals to allow for higher mineral concentrations. This decreases the need for makeup water but does not eliminate it.
The term “cycle of concentration” is used to determine the mineral concentration of the coolant water in relation to the concentration of raw makeup water. For example; if the concentration of the coolant water is four times the concentration of the makeup stream the cycles of concentration are four. In other words, it is a relative measurement of the mineral concentration in the water coolant.
The table below clearly shows the cost benefit of using high cycles of concentration. It also shows the effect of diminishing returns especially for more than 5 cycles. It is also important to note that if the initial mineral content of the makeup water is high, lower cycles can be used.
| Cycles | Bleed (m3/day) | Makeup (m3/day) | Annual water cost* | % Reduction water cost | % Reduction inhibitor cost |
|---|---|---|---|---|---|
| 1.5 | 163.53 | 245.29 | $70,956 | 0 | 0 |
| 3 | 40.88 | 122.65 | $35,478 | 50.0 | 75.0 |
| 5 | 20.44 | 102.21 | $29,565 | 58.3 | 87.5 |
| 8 | 11.68 | 93.45 | $27,031 | 61.9 | 92.8 |
| 10 | 9.08 | 90.85 | $26,280 | 62.9 | 94.4 |
Measuring the cycles of concentration
The cycles of concentration can be measured either chemically or by performing a mass balance over the system. The chemical measurement may be performed according to the following formula:

Cycles of concentration formula.
The cycle measurement can also be performed using the mass balance according to:
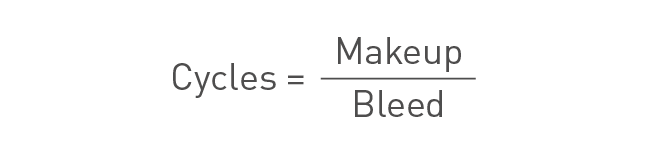
Cycles of concentration formula for mass balance.
Where: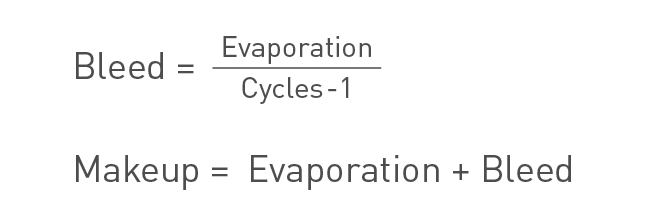
Monitoring and adjusting mineral concentration
It is essential to determine the maximum mineral concentration that is allowed before scaling occurs. This value is then used to adjust the blowdown rate to set the maximum amount of cycles.
The Langelier saturation index (LSI)
The LSI uses the calcium concentration, alkalinity, conductivity (in TDS), and water temperature to determine the maximum stabilization pH of calcium. Chemical treatment is then used to increase the solubility of calcium carbonate to be able to reach higher cycles. This way, using a chemical treatment programme, an LSI of about +3 can be reached without significant scaling and the LSI is then controlled by the amount of system bleed/blowdown.
Practical ozone Scaling Index (POSI)
To monitor and control scaling when using ozone treatment the POSI index was developed by Pryor and Fischer in 1993. It gives the maximum operation conductivity for the cooling tower to avoid scaling and it takes the reduced amount of dissolved calcium (by using ozonation) into account. The index is explained in the formula below:

Practical Ozone Scaling Index (POSI).
POSI example
To further clarify how the POSI can be used an example makeup water quality is given in the table below and POSI is calculated:
| Parameter | Value | Unit |
|---|---|---|
| pH | 8.4 | |
| Conductivity | 130 | µS |
| Calcium hardness | 30 | ppm CaCO3 |
| Magnesium hardness | 10 | ppm CaCO3 |
| Sodium | 10 | ppm Na |
| Chloride | 7 | ppm Cl |
| Total alkalinity | 39 | ppm CaCO3 |
| Temperature | 13 | ⁰C |
Which gives:
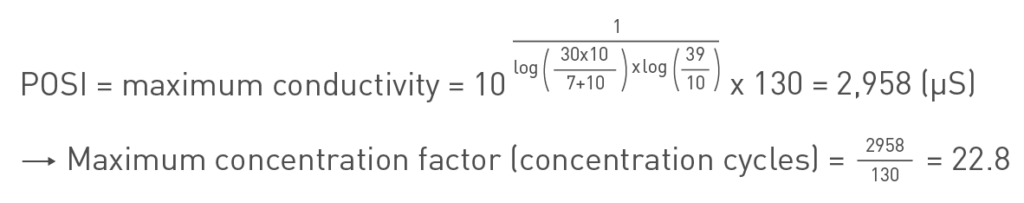
In other words, when applying ozone treatment to this makeup water the maximum conductivity may reach a value of just below 3000 µS to avoid scale formation. This enables the process to run at almost 23 cycles. A chemical programme for the same makeup water quality would enable a process to run at about 10 cycles.
Ozone dosing and process design
In the following section a few simplified mathematical relationships are presented for estimation of the ozonation equipment design. The amount of required ozone is based on the recirculation rate of the cooling tower water. The recirculation rate may be obtained from system volume and turnover period:
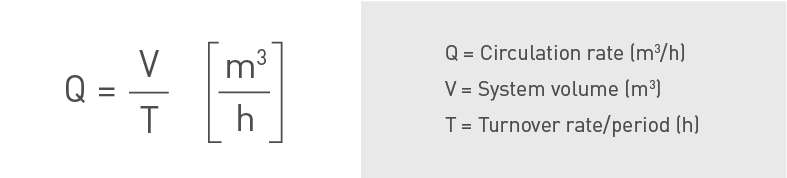
Cooling tower circulation rate.
Typical recommended values for required ozone concentrations for different sections of the cooling tower are listed in the table below:
| Process section | Recommended value range [ppm] |
|---|---|
| Cooling tower basin | 0.025 – 0.250 |
| Recirculating pump inlet | 0.075 – 0.150 |
| Heat exchanger inlet | 0.040 – 0.080 |
| Return line to tower | 0.010 – 0.040 |
Ozonation of about 0.2 ppm is usually provided to a side stream of the main flow. The contacting equipment allows for about 90 % dissolution efficiency of the generated ozone. However a dissolution efficiency of 80 % may be used for extra margin. Furthermore the ozone generator capacity decreases over time.
Hence, a decrease in capacity of 10 % over the course of two years can be used (again for extra margin). To estimate the required ozone production capacity, “ṁO3”, of the generator the following formula may be used:
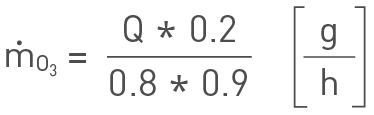
Ozone mass flow rate.
Hence, for example a system volume of 500 m3 and turnover period of 30 min requires an ozonation system with a capacity of about 280 g/h. Note that dosing requirements have to be adjusted with respect to important factors like e.g. water temperature and quality for optimal efficiency. Moreover ozone dosing should not exceed 10 g/m3 makeup water.
Measuring and regulating ozone demand
ORP-measurements should be made continuously to provide adequate ozone dosing to the system. Note that ORP probes are prone to fouling by e.g. calcium carbonate levels. However cleaning is simple, albeit essential. This way, excessive ozone generation is provided which results in energy savings and elimination of corrosive effects from excessive ozone.
Ozone compatible materials
Listed below are materials considered to be suitable with ozonation processes:
| Piping: | 316 Stainless steel Teflon/PTFE Kynar/PVDF |
| Vessels: | 316 Stainless steel (welds to be ground smooth internally) |
| Gaskets: | Teflon/PTFE FPM/Viton |
Ozone compatible chemicals
Depending on water quality and process type, in some cases it might be beneficial to use chemicals together with ozone to some extent. It is important however not to interfere with the treatment programme integrity as well as to only use chemicals that maintain their function and stability in combination with ozone. Listed below are examples of chemicals that have been shown to be ozone compatible:
- PBTC, scale and corrosion inhibitor.
- Molybdate, corrosion inhibitor for soft water.
- Silicate, corrosion inhibitor at calcium concentrations <200 ppm.
- TTA/BTA, copper and brass alloy protection.
- Zinc based chemicals, corrosion inhibitors.

