CIP & process equipment sanitation
Shorten your CIP-process and lower your maintenance costs
Throughout the industrial sector, food & beverage, dairy facilities, process industry and pharmaceutical plants, major amounts of water, energy and chemicals are spent in order to maintain clean and sanitized process equipment.
Equipment such as tanks, pipes, valves, heat exchangers and filling machines need to be kept free from unwanted microorganisms, biofilm, scale and chemical residues. Failure to maintain clean process equipment may lead to the following:
- Spoiled final product
- Inefficient heat exchangers
- Heavy maintenance
- Increased demand on chemicals, water & energy
Clean-in-place
CIP basics
Clean-in-Place (CIP) is one of the most common unit operations throughout the industrial sector and is crucial in order to clean process equipment between different batches. Today, these processes are fully automatic with central CIP stations installed which serve the whole plant’s process equipment cleaning and sanitation needs. They enable cleaning cycles inside pipes and tanks which are otherwise inaccessible for plant staff. Typically it includes the following steps:
| CIP cycle step | Purpose | Cleaning agent | Challenges |
|---|---|---|---|
| 1. Pre-rinse | Remove majority of particles, organic material before cleaning agent. | Water. | High water demand, wastewater loads. |
| 2. Caustic or acid cleaning | Clean organic material and particles which have adhered to internal surfaces. Acid cleaning may also remove inorganic particles to avoid scaling. Remove surfaces that enhance microbial growth. | Sodium or potassium hydroxide, various acids. | Chemical handling, costly. |
| 3. Rinse | Rinse away caustic or acid in order to avoid product contamination. | Water. | High water demand, wastewater loads. |
| 4. Sanitation & disinfection | Ensure microbial (bacteria, viruses, algae) free process equipment. Even a few microbes may cause infections of final product. | Chlorine based agents, peracetic acid (PAA), hot water, steam, iodophores. | Ensuring complete removal of bacteria. High costs. |
| 5. Final rinse | Rinse away sanitation chemicals to avoid contamination of brew. | Water. | Adds time and water consumption to the CIP cycle. |
Typical CIP phases in process equipment cleaning and sanitation.
Ozone CIP
Ozone technology presents a novel approach to sanitizing process equipment with significantly lower water, chemicals and energy demand. Ozone completely replaces costs and handling of traditional sanitation methods using chemicals or hot water. Ozone leaves no residual chemicals after use which means final rinse is not required. It is also possible to apply ozone directly following cleaning – ozone rinsing. This enables a 3-stage CIP instead of 5 cycles. The figure below illustrates CIP improvements that can be made by replacing chemicals and utilizing the unique characteristics of ozone. It shows how a 5-stage CIP cycle can be reduced to a 4-stage cycle.
Ozonetech RENA Vivo systems are a perfect fit for CIP applications, which offer a complete turn-key solution which can be integrated easily into existing plants or connected during construction of new facilities.
Hot water sanitation is commonly used where chemical use is to be avoided. However, it is time consuming and exerts strain on the mechanical equipment during expansion and contraction during heating and cooling, causing leakages and expensive maintenance. Since ozone leaves no residues, it is a viable cold sanitation method in lieu of hot water use, which saves major amounts of energy and associated costs. This concept is illustrated below:

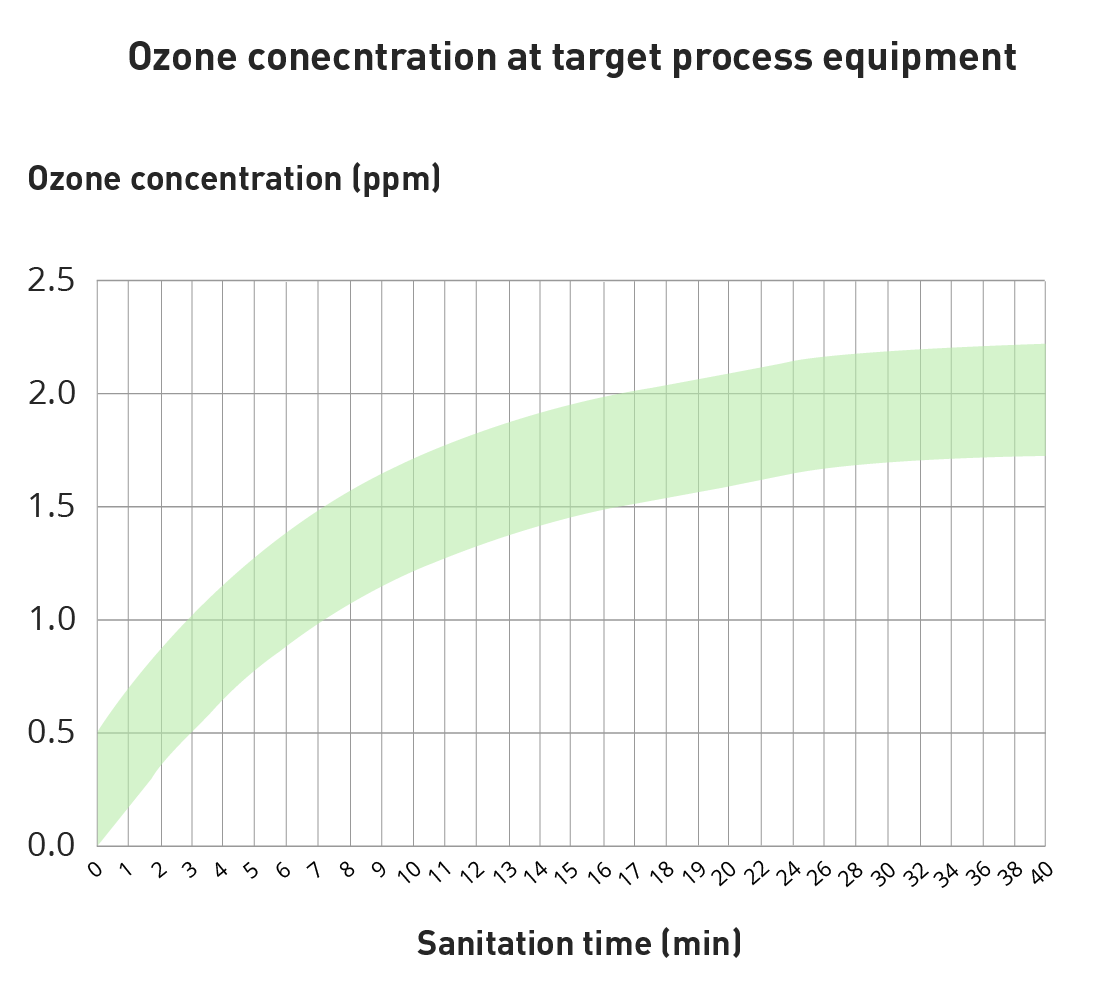
Ozone is produced in-situ and on demand, which means that it is generated and applied when it is needed. This is the key characteristic of ozone compared traditional chemical agents. The figure below shows the ozone concentration over time for a facility. The green area exemplifies the range of ozone concentration applied on the process equipment. As can be seen below, the ozone contration increases over time and reaches adequate levels for disinfection in just a few minutes.
The sanitation cycle is complete in 5-15 minutes depending on the amount of microorganisms and type. Since ozone is approximately 10-1000 times more effective than other chemicals against bacteria, mold and viruses, the sanitation can be ended in only a few minutes, saving important down-time for any type of facility. Typical target ozone concentration is 1 ppm for 3 log reduction of all types unwanted microorganisms in 15 minutes. Please read more about ozone disinfection mechanisms at our Disinfection application section.
The following video shows our RENA Vivo A-series as an integrated part of a CIP process, in the following example a brewery:
Clean-out-of-place (COP)
While CIP refers to cleaning and sanitation of the interior of process equipment, COP refers to surface sanitation of tanks, pipes or floors. Typically, foaming agents are used to aid the visual inspection of cleaned versus non-cleaned areas. Ozone presents and opportunity to avoid exposure of chemicals during COP operations by employing an ozone system to produce ozone on demand and in-situ. Ozone use provides a clear indication since it leaves a gentle odor after use which is an indicator of successful sanitation.
Biofilm removal
Due to the strang disinfection power of ozone, existing biofilm can be removed by ozonating over an extended time period, allowing ozone to break down cell walls and organic material. Biofilm may occur in systems where insufficient sanitation or cleaning has allowed for biological growth of bacteria on organic material left from production. In such cases, ozone is an excellent way to remove the biofilm and prevent future growth.
Cooling towers and closed water systems
These types of applications differ in some respects from CIP applications. In closed cooling systems or cooling towers, large volumes of water circulate with very long hydraulic retention. In these instances, ozone concentrations below 0.2 ppm suffice to prevent microbial growth. With large hydraulic retention times, existing biofilm can be degraded effectively.
Applicable industries
Ozone sanitation of process equipment is especially valuable for breweries(external site), dairy facilities (external site) off-shore and food production (external site). Food production also has an extended use in terms of rinse of fresh-produce.

