COD & BOD treatment with ozone
Ozone and AOP solutions for high COD & BOD concentrations
The chemical oxygen demand (COD) is a measure of wastewater and water quality that can be used as in indirect measurement of the amount of oxidizable compounds. COD evaluates all chemically oxidizable substances and can be directly related to the true oxygen demand of the effluent if released in the environment. It is therefore an effective tool for getting a picture of the environmental impact from a possible discharges of wastewater into the environment.
BOD, short for “Biological Oxygen Demand”, also measures the oxygen demand in the water but bases the oxygen demand on the amount of oxygen consumed as microorganisms are responsible for the oxidation process (instead of a chemical oxidant as in the case of the COD measure).
In most cases COD concentrations are higher than BOD in wastewater process since not all organic material are biodegradable. Typical COD range for chemical process wastewaters is 200 – 40 000 mg/L and domestic wastewaters in the range of 100 – 450 mg/L. The image below shows a brief overview of various types of compounds and how they can be treated with ozone.

Other industry effluents such as breweries, diary, iron & steel, pulp & paper and mining also produces process water that contain high COD levels above >1000 ppm. Organic COD in wastewater can be classified into non-biodegradable and biodegradable COD as can be seen in the table below.
Non – biodegrable COD
The non-biodegradable COD is a result of soluble substrates that are found in the effluent and escape the wastewater treatment plants in small concentrations as micro pollutants. Some of the examples include pharmaceuticals (for example: diclofenac, paracetamol and propofol), persistent organic pollutants (pesticides, industrial chemicals and hormones) and other molecules including fluorinated and bromated organic compounds. The table below shows some typical compounds and their characteristics in terms of oxygen demand and ozone treatment demand.
| Compounds | BOD5/COD ratio | COD:O3 ratio |
|---|---|---|
| Pharmaceuticals | 0.1 | 6:1 to 3:1 |
| Persistant organic pollutants | 0.2 | 5:2 to 2:1 |
| Aldehydes | 0.3 | 7:1 to 4:1 |
| Amines | 0.5 | 4:1 to 3:1 |
A low BOD:COD ratio represents a compunds that is not readily biodegradable.
Biodegradable COD
The biodegradable COD is further divided into readily biodegradable (soluble compounds such as volatile nutrients, fatty acids, sugar, selected alcohol and proteins) and slowly biodegradable compounds. Slowly biodegradable compounds are composed of particulate, colloidal and complex organic molecules.
| Compounds | BOD5/COD ratio | COD:O3 ratio |
|---|---|---|
| Fatty acids | 0.4 | 2:3 |
| Nutrients (ammonia, organic phosphate) | 0.6 | 7:1 |
| Proteins | 0.7 | 6:2 |
| Alcohol | 0.9 | 5:3 |
| Sugars | 0.7 | 4:2 |
Typical ranges of concentration of COD and BOD5 can be found in the table below:
| Oxygen demand type (mg/L) | Domestic wastewater | Food & beverage | Chemical & pharmaceutical |
|---|---|---|---|
| BOD5 | 200-300 | 450-1100 | 250-1000 |
| COD | 450-700 | 1450-2200 | 2000-18750 |
Ozone
Ozone is a powerful oxidant and is easily soluble in water. It is a residual free treatment technology that has the potential to eliminate complex organic pollutants. More detailed information can be found here. In the following sections a few key opportunities of ozone based treatment is presented to treat organic carbon in wastewater effluents.
Ozone with AOP
AOPs are advanced oxidation process utilizing extremely reactive species for the destruction of target pollutants. Formed radicals have higher oxidation potential than ozone and a reaction rate which is about a million times higher, thus leading to lower contact time and footprint. AOP can also be applied to achieve complete- or partial oxidation of the pollutants in the treatment process. A few examples of reaction mechanisms in formation of hydroxyl radical with ozone are shown below.
| Ferric oxide | Fe3++ O3 → (FeO)2++OH– + O2 + H+ |
|---|---|
| Hydrogen peroxide | O3 + H2O2 → OH– + O2 + H2O |
| Ultraviolet | O3 + H2O → O2 + H2O2 (In the presene of UV) 2O3 + H2O2 → 2OH + 3O2 |
Other AOP methods include combination of Titanium dioxide and UV techniques to produce hydroxyl radicals.
An example from Ozonetech pilot facility: Removal of COD and BOD using ozone in industrial wastewater streams is found below:
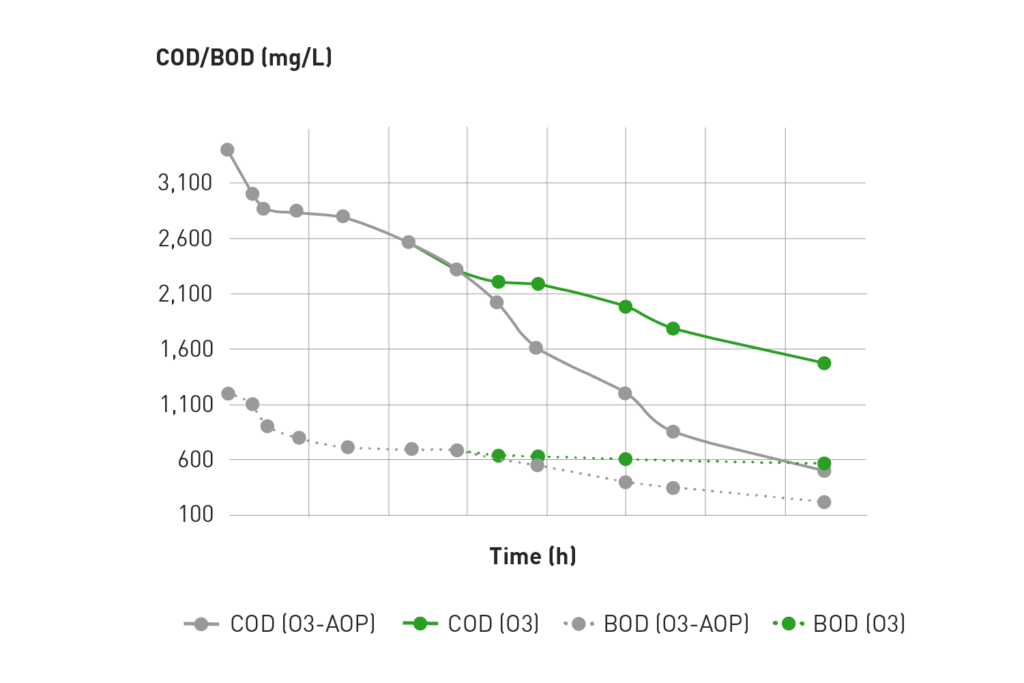
Ozone-AOP is beneficial in situations where ozone alone cannot achieve complete oxidation of wastewater compounds. In these instances, AOP can be successfully applied to augment the reaction kinetics to remove even the most complex substances. This can clearly be observed in the above graph.
Ozonetech has extensive experience in treating complex organic streams from the process industry, pharmacautical industry and and & beverage production. We offer pilot projects and full scale ozone-based treatment systems. More information about feasibility studies and pilot project can be found here.

